Apollo 17 Lunar Soil Sample Still Surprises After 50 Years
University of Houston Student Discovers Unexpected Mineral
Monica Martinez, Junior year student at University of Houston, with her poster on the lunar soil sample.
NASA Photo AS17-137-20990 showing the orange regolith at this site, complete with the astronauts' boot-prints.
Lunar soil samples collected from the last moon landing nearly 50 years ago are yielding a few surprises for a new generation of researchers. When an undergraduate at the University of Houston (UH) landed the opportunity to examine a sample of the fine-grained regolith (essentially inorganic topsoil) collected by the Apollo 17 astronauts in 1972, she and her PI were surprised to find minerals not expected in the regolith.
The Junior year student, Monica Martinez, had attended Houston Community College (HCC) for two years before beginning Mechanical Engineering studies at the University. She had been selected by Mr. Bartlett Sheinberg of HCC to participate in his REEMS REU program which included undertaking a summer research project at UH under the tutelage of Dr. James Meen of the Department of Chemistry and Texas Center for Superconductivity at UH. When at UH, she was provided a portion of a lunar soil sample by Col. Dr. Donald Barker who completed his Ph.D. in Planetary Geology at UH in 2018. She was excited to have the chance to examine the sample in the electron microscope. “How could I say no to working with moon rocks?” she recalls. It led to an exciting discovery and she has two published conference proceedings to date on this second major project with Dr. Meen.

Professor James Meen, Research Associate Professor, Department of Chemistry Task Leader, Material Characterization Facility, TcSUH, with the field emission SEM.
Regolith with a TEM grid for scale. The gaps between copper bars are 58 µm across (hence the scale bar is 58 µm). The whole grid is 3 mm across.
Apollo 17 landed at Taurus-Littrow on the southeastern rim of Mare Serenitatis (30° 44´ 58.3¨ E, 20° 9´ 50.5¨ N), a site expected to contain explosive volcanic, dark mantle deposits. Samples studied by Martinez were collected from the top 10 cm of orange regolith at Station 4 on the rim of Shorty Crater. Apollo 17 was the last Apollo mission to land men on the Moon and the only one to carry a geologist.
Martinez had already been characterizing materials using the lab’s Field Emission SEM, a model JSM-6330F. Well-documented studies exist on lunar soil, so when she discovered a Calcium Sulfide (CaS) grain, she knew it indicated a potentially novel discovery.
Martinez indicates the Apollo 17 landing site on a ballroom-sized map of the moon at the Lunar and Planetary Science Conference (LPSC).
The 5-10m deep regolith that covers most of the lunar surface is dominantly made up of materials that are derived from pulverized lunar bedrock, micrometeorite bombardment, and volcanic glass, modified by H and He atoms in the solar wind and by cosmic rays (processes collectively known as gardening). These discoveries provide a novel perspective on the inner workings of the gardening process within the regolith. Some regions of the regolith contain grains, comprising a small modal proportion overall, of minerals not expected to have formed in the original lunar igneous rocks. They include calcium sulfide, sodium-potassium chlorides (“mare-salt”), and elemental antimony.
Her discovery is, “Completely new. Calcium sulfide is very rare on the earth and the moon. It is also a rare high-temperature meteorite mineral. Other lunar occurrences are believed to be fragments derived from impactor meteorites and not in the igneous rocks.” Martinez says. “Other grains of CaS have a slightly different chemical composition. Additionally, the unusual habit of these grains and the phase relations of the minerals indicate that the CaS sublimated from a vapor onto a solid surface and grew into a void. Many of the chloride grains have strong cubic habit and are solid solutions of NaCl and KCl which is consistent with their growth from a chloride liquid, again on a solid surface and again into a void.”
“The moon provides a harsh, utterly non-Earth-like environment. Although vapor phase processes have long been proposed, Monica’s discoveries show an extra level of unexpected weirdness. Following meteorite impact, highly corrosive high-temperature gases including metals such as Ca as well as Na, K, S, Sb, and Cl can be generated, if only for a short time,” says Meen.
Use of the SEM quickly became a regular part of Martinez’s earlier research work on other more worldly samples, including use of Energy-dispersive X-ray Spectroscopy (EDS) for elemental analysis. She used X-ray analysis to see the distribution and quantity of the elements in the CaS grain and surrounding phases, or material.
While Martinez pursues her undergraduate degree in Mechanical Engineering, she already plans to turn this work into her Honors Thesis and pursue a Master’s and eventually PhD in Aerospace Engineering. She feels that the combination will be building a bridge between Mechanical Engineering and Aerospace Engineering, fundamental to the communication of scientists and engineers in the aerospace industry.
Little did she know that the influence of the clear night skies at her grandparents’ farm in the country, where she could see the moon and stars so brightly shining above, would lead her to examining lunar dust and making a breakthrough discovery early in her path to her career.
The Science: Lunar Soil Sample Examined by SEM
Sulfides
SEM image of CaS grain in lunar soil.
An SEM image of CaS grain in lunar soil taken by Monica Martinez, a student of Dr. James Meen at University of Houston. According to Meen, “What is interesting about the CaS grain (top left) is that there are those “tentacles” coming off it. If this CaS is oldhamite (yet to be determined), it is cubic and that growth habit is non-equilibrium in terms of surface energy. Slow growth would have yielded a euhedral shape (given it is similar in crystal structure to galena, probably as cubes). I don’t think it is ever seen in euhedral crystals on earth or in meteorites as it grows with other minerals around it.” He adds that there are pictures of terrestrial oldhamite at
https://www.mindat.org/min-2970.html.
The sulfide grew rapidly and into an open space. The lunar regolith is about 50% pore space so it likely grew into a pore. CaS melts at around ≈2500°C. The melting temperature of the glass it is growing on is about 1000°C. At 1500°C, the regolith would be 100% liquid. There would be neither pores nor solid surfaces at 2500°C so the CaS did not grow from a liquid. It sublimated. A possibility is that Ca gas generated at >2000°C by micrometeorite impact entered a pore surrounded by glass at <1000°C (also heated by impact but farther away) and from which S had evaporated and they reacted to precipitate CaS. There was also Na and K introduced and they dissolved in the sulfide. This pore had no Cl gas for whatever reason.
Chlorides
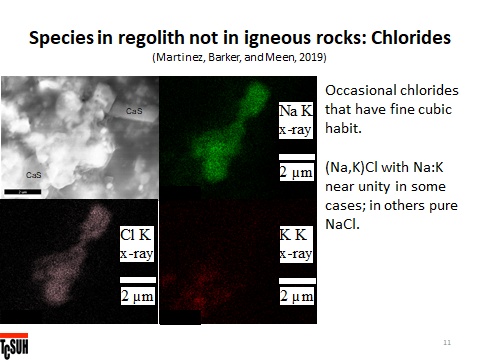
Meen says, “The relatively large cluster in the middle is a NaCl-KCl solid solution (Na>K) as seen from x-ray maps. Unfortunately, the chlorides are easily damaged by the beam. The cube at top has black spots that are electron pits and the big piece started to melt in the beam. NaCl and KCl on earth form naturally almost entirely from seawater at temperatures of, say, 40°C. There is no mutual solubility of Na and K in the chlorides below 200°C. Monica has chlorides near Na:K=1:1 which means they crystallized from a liquid above 650°C. The cubes indicate that they grew into a void and again they are on glass surfaces in regolith. NaCl and KCl boil around 1450°C. Best guess – Na and K were formed by impact and moved into pores at lower temperature that contained Cl evaporated from glass. The alkalis and Cl reacted and condensed as a liquid on the surface at <1000°C, cooled, and crystallized cubes of chlorides. Note that this field of view contains CaS as well. And that CaS does not contain alkalis. So… Ca, Na, and K were all introduced into a pore containing Cl and S and the alkalis reacted with the Cl and the S with the Ca because that is what thermodynamics requires. The process is not equilibrium but it can do some preferential match-making.”
Native Antimony
The observation of elemental antimony was also a surprise. Although Sb is also a relatively volatile element (melts at 630°C; boils at 1635°C), it has extremely low concentrations in lunar rocks (a few ppb at most). Creation of Sb vapor required extracting the Sb from a relatively large volume – the grains of Sb represent a concentration factor of a billion.
Additional Reading: