Why Use EDS Analysis for Li-Ion Batteries?
Before lithium-ion (Li-ion) battery commercialization, and subsequent domination of the energy storage market, rechargeable battery chemistries were fraught with limitations. Low energy density, memory effects, high self-discharge rates, and toxicity issues were commonplace. Li-ion batteries, by contrast, delivered higher voltages (around 3.6V per cell). They also offered low self-discharge, rapid charging capabilities, and reduced maintenance. These properties ensured the quick adoption of Li-ion chemistries in portable technologies, fuelling enormous growth through the 1990s. Some experts credit the technology as the foundation of wireless tech. Now, Li-ion batteries are ubiquitous in modern society, and demand is ever-growing.
This growth in demand presents a constant need to optimize performance, safety, and longevity. One technique for achieving these improvements is energy-dispersive X-ray spectroscopy (EDS) analysis. Our blog post delves into why EDS analysis is indispensable for Li-ion battery research and development.
Non-Destructive Elemental Analysis and Mapping
One of the most compelling reasons to use EDS analysis for Li-ion batteries is its ability to perform non-destructive elemental analysis and mapping. EDS can analyze various battery components such as anodes, cathodes, and separator materials.
EDS mapping is instrumental in revealing the distribution and interfaces between different layers and components within a Li-ion battery. Understanding these interfaces is key to enhancing battery performance and longevity. For instance, the interface between the anode and the electrolyte must be well-understood to prevent issues such as dendrite formation, which can cause short circuits and battery failure.
In figure 1, a cross-section of a cathode material was prepared with JEOL’s cross-section polisher. The overall EDS spectrum of the area, individual EDS element maps, and an overlay of these elements is shown.
By providing a detailed elemental map, EDS enables researchers to visualize the distribution of elements and identify any inhomogeneities or defects. This insight is crucial for optimizing the materials and interfaces within the battery, leading to more reliable and efficient energy storage solutions. Additionally, EDS provides this detailed elemental mapping without damaging the sample. The non-destructive nature of EDS ensures that the samples remain intact for further testing and analysis, thereby preserving valuable material and enabling comprehensive studies.
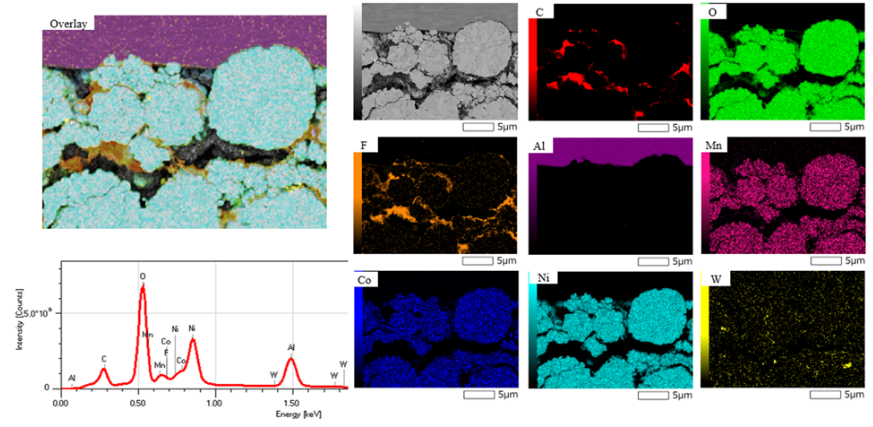
Figure 1: Individual EDS elemental maps, overlay map, and overall EDS spectrum of a cross-section of cathode material.
Correlating Electrochemical Behavior with Microstructural Changes
Another significant advantage of EDS analysis is its ability to be combined with high-resolution imaging in scanning electron microscopy (SEM). This combination allows for the correlation of electrochemical behavior with microstructural changes during charge and discharge cycles. Understanding these correlations is crucial for improving battery performance and addressing issues such as capacity fade and thermal runaway.
High-resolution imaging coupled with EDS provides a comprehensive view of the battery’s microstructure and elemental composition. Researchers can observe how the microstructure evolves over time and how it affects the battery’s electrochemical properties. This holistic approach leads to a deeper understanding of the mechanisms driving battery performance and degradation, paving the way for innovative solutions to enhance battery life and safety.
Air-Isolated Workflow for Sensitive Materials
Lithium oxidizes extremely quickly, making lithium-containing materials a challenge to analyze. Exposure to the atmosphere can alter their composition, leading to inaccurate results. JEOL addresses this issue by offering an air-isolated workflow using a transfer vessel and an inert gas environment for each of our SEMs. This setup prevents atmospheric exposure, allowing for pristine sample analysis.
Figure 2 shows a transfer vessel setup. A sample can be mounted to the sample holder while inside a glove box and then capped with an O-ring seal. The capped sample holder can then be inserted into the specimen exchange chamber of a SEM and the exchange chamber can be evacuated. Once under vacuum, the cap can be removed by screwing the knob assembly into the cap and pulling up. The cap remains in the exchange chamber while the sample is imaged. From there, the sample can be recapped before removing the sample from the SEM.
The air-isolated workflow ensures that sensitive materials remain unaltered during the transfer and analysis processes. This is particularly important for studying materials prone to rapid oxidation or other atmospheric reactions, such as lithium. By maintaining the integrity of the samples, researchers can obtain accurate and reliable data, which is essential for advancing battery technology.
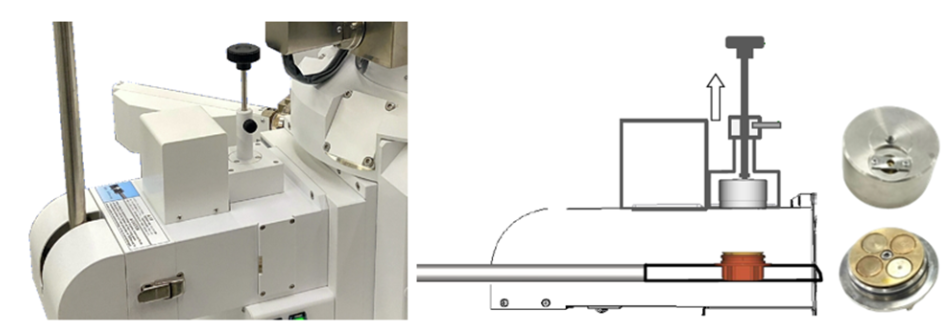
Figure 2: One of JEOL’s air-free transfer solutions. In this case, the sample is mounted to a transfer vessel that is capped in a glove box. The cap is then removed under vacuum in the specimen exchange chamber of the SEM. The sample can be recapped and brought back to the glove box without air exposure.
Detection of Lithium with Windowless EDS
Lithium poses significant challenges for detection and quantification. Firstly, lithium which is bonded to oxygen will not produce many X-rays. Moreover, a standard EDS has a thin polymer or silicon nitride window to protect the silicon drift detector (SDD). This window will absorb very low-energy X-ray signals. Even if Li-containing samples are metallic and kept isolated from the environment, a windowed EDS detector cannot directly detect the characteristic X-rays of lithium (Li-K 54 eV).
Windowless EDS detectors, such as JEOL’s GatherX detector, allow for the detection of ultra-low energy X-rays. These detectors are especially adept at analyzing elements with low atomic numbers like carbon, fluorine, and, critically, lithium, which is why they are crucial for pinpointing the localized chemical makeup of battery components. Lithium’s distribution directly impacts the battery’s performance, efficiency, and safety.
Figure 3 displays an area of a cross-section of a pristine Li metal sample. It shows a sharp Li-K peak, with differences between the inside and the near surface areas of the metal. EDS analysis allows researchers to observe subtle changes in lithium distribution within battery materials. This capability is essential for learning about how lithium migrates during charge and discharge cycles, which in turn helps design batteries with improved charge retention and lifespan.
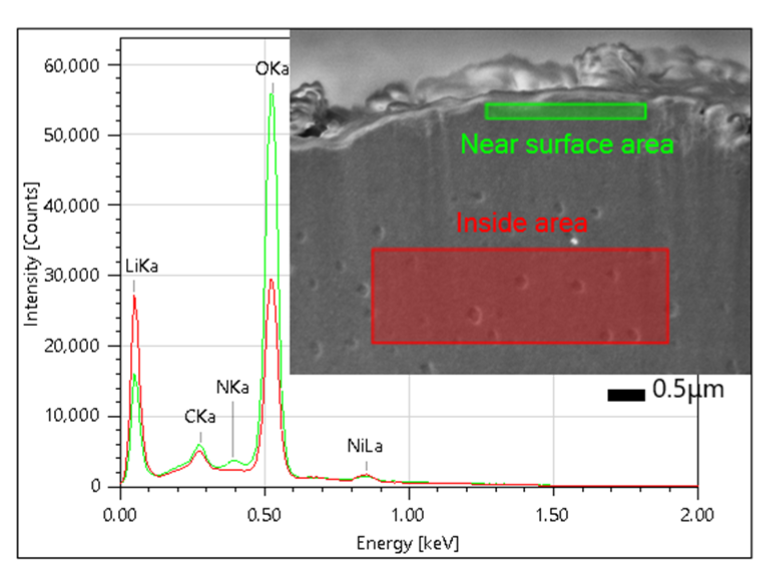
Figure 3: The area analysis result of a pristine lithium metal cross-section. It shows a sharp Li Ka peak, with differences between the inside and the near-surface areas.
Want to Learn More?
EDS analysis is an indispensable tool for Li-ion battery research and development. Its ability to perform non-destructive elemental analysis and mapping, coupled with high spectral resolution and air-isolated workflows, makes it invaluable for studying battery components and their interactions. By providing detailed insights into the distribution of elements and microstructural changes, EDS enables researchers to design better performing and longer-lasting batteries. As the demand for advanced energy storage solutions continues to grow, the role of EDS in Li-ion battery research will only become more critical.
At JEOL USA, we are committed to advancing the field of lithium-ion battery research with cutting-edge analytical techniques like EDS. Through leveraging our expertise and innovative technologies, researchers can trust the accuracy of their quantitative EDS data, ensuring that every insight leads to tangible improvements in battery performance and safety.
Discover more about how our solutions can enhance your research and trust in your quantitative data by visiting our comprehensive guide.